Joud Odeh
Development of sleep models for the diagnostic prediction of Alzheimer's disease
Joud Odeh talks about her Master's thesis in which she analyzed the sleep behavior of patients with different stages of Alzheimer's disease.
Motivation
Due to increasingly aging populations caused by extended life expectancies and low population growth rates, research tackling age-related neurodegenerative disorders, such as Alzheimer’s disease (AD), has gained importance and momentum in the past few years [1]. Within the realm of AD research, a growing interest is evident in developing digital biomarkers for early disease detection and the subsequent integration of appropriate preventive measures. Such measures result in improved disease management, enhanced patient quality of life, and an alleviation of burden on both healthcare institutions and caregivers alike [2–4].
There have been a number of studies suggesting a bidirectional relationship between sleep disturbances and AD [5]. Therefore, exploring sleep disturbances and variations in sleep patterns as potential digital biomarkers for AD presents an opportunity for early disease intervention and slowing down disease progression [6]. Common methods for monitoring sleep behavior include self-reported sleep assessments, such as sleep questionnaires, sleep diaries, and clinical interviews. Despite the importance of these existing measures, relying mainly on informant feedback, clinician guidance, and self-reporting may introduce significant subjectivity and the potential for human error. Therefore, the integration of such established standards with nonobstructive, easily operable wearable digital devices for sleep monitoring, such as actigraphy (accelerometry), as well as other remote monitoring technologies (RMTs), allows measurements to be recorded in a home setting and relayed directly to healthcare professionals. This facilitates the continuous monitoring of abnormal sleep behaviors and aids in the early identification of symptoms relating to AD [7,8].
Study design: statistical modeling and analysis of sleep behavior
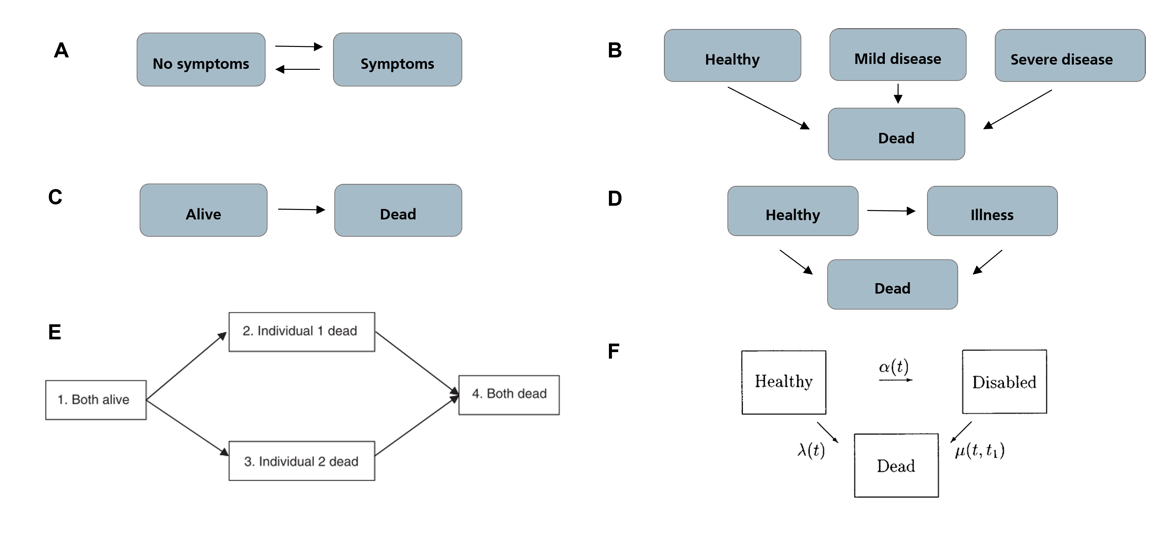
For the modeling and analysis of sleep, well-established time-to-event analysis approaches are commonly employed. The most common approaches include stochastic modeling approaches, such as Markov chains or Markov models, and survival analysis, which aims to predict the time until an event occurs. A Multi-state model (MSM) is a model that describes a stochastic process that, at any given time, can exist in one of several distinct states. MSMs are commonly used in modeling longitudinal failure time data. They are considered extensions of the well-established Kaplan-Meier and Cox models for survival analysis with not only two (event has or has not occurred) but potentially a higher number of distinct states. [9]
In medical applications, MSMs are often employed to model disease progression, where each state represents a different disease stage, and a visit from one state to the next is referred to as a transition (see Figure 1).
In our work, we employed MSMs for the modeling of sleep cycles (Figure 2) to understand the discrimination between diagnostic groups with respect to sleep behavior. We also analyzed: 1) precomputed features of remote sleep monitoring devices, and 2) scores from subjective sleep questionnaires, namely ESS [12] and PSQI [13], to evaluate the predictive performance of AD vs. non-AD groups. The study employed sleep stage data from actigraphy and wearable EEG devices to build multi-state models of sleep cycles across the groups. Relevant patterns and hazards from these models were further investigated. Furthermore, precomputed sleep quality scores from wearable devices such as the number of nocturnal awakenings and share of sleep time spent in rapid-eye movement (REM) or deep sleep stages, as well as sleep questionnaire scores, were compared across groups.
Results and conclusion
The first part of our work focused on the multi-state modeling of sleep stage data derived from the Fitbit and DREEM devices employed in the RADAR-AD study. This study is particularly interesting as it also included participants from the preclinical AD category. For every device, group-level MSMs (grouped by AD stages of increasing severity, namely: Healthy Controls (HC), Preclinical AD (PreAD), Prodromal AD (ProAD), and Mild to moderate AD (MildAD)) were fitted, analyzed, and the patterns compared. Derived patterns, including sojourn times (time spent in a state at a time) and transition probabilities (probability to transition from one state to another), indicated that AD patients often spend longer times in the lightest sleep state, namely N1, are likely to experience nighttime awakenings, and tend to spend less time in deep and REM sleep stages. The statistically significant differences in sleep patterns observed even in the preclinical groups also reveal that sleep and circadian rhythm disruptions can occur in very early disease stages [5, 6, 14, 15].
For the second goal of our study, a statistical analysis of sleep questionnaires and sleep scores was performed. Generally speaking, statistically significant differences were found among ESS and PSQI scores not only between AD and non-AD participants but also within AD groups of varying severity. These are interesting observations, as they show that subjective sleep measures may discern between groups, even without using sleep monitoring devices. For the comparison of the Fitbit precomputed features, Healthy controls and Prodromal AD features showed significant differences in deep sleep percentages and REM sleep percentages. For the pairwise comparison between healthy controls and Mild AD participants, deep sleep percent, REM sleep percent, and awakenings differed significantly.
Based on our observations from both subjective and objective sleep monitoring methods, we concluded that detectable variations in sleep behavior and sleep quality exist across AD groups. Our findings confirm the potential of sleep behavior for early AD detection, especially when coupling remote measurement devices with subjective measures such as sleep questionnaires. Although it is an interesting and well-researched topic, more needs to be done to develop quantifiable prognostic measures based on sleep. Further feature importance and sleep modeling may be necessary to understand sleep and AD better and develop standardized metrics to be followed by medical practitioners [16]. If detected in the early stages, and due to the bidirectional nature of AD and sleep, pharmacological and non-pharmacological interventions that target sleep quality may be employed to hinder progress, such as behavioral measures, bright light therapy (BLT), and melatonin supplementation. [17]
References:
[1] H. Niu et al. “Prevalence and incidence of Alzheimer’s disease in Europe: A metaanalysis”. In: Neurología (English Edition) 32.8 (Oct. 2017), pp. 523–532. issn: 2173-5808. doi: 10.1016/J.NRLENG.2016.02.009.
[2] Disease Management | AMCP.org
[3] Radar-AD | Radar-AD
[4] Reisa A. Sperling, Clifford R. Jack, and Paul S. Aisen. “Testing the Right Target and the Right Drug at the Right Stage”. In: Science translational medicine 3.111 (Nov. 2011), p. 111cm33. issn: 19466234. doi: 10.1126/SCITRANSLMED.3002609.
[5] Mahnoosh Kholghi et al. “The association between sleep restlessness and neuroimaging biomarkers of Alzheimer’s Disease in mid-life to older adults and patients”. (2023). doi: 10.1002/alz.062017
[6] Ludovica Rigat et al. “Dysfunction of circadian and sleep rhythms in the early stages of Alzheimer’s disease”. In: Acta Physiologica 238.2 (Mar. 2023). issn: 1748-1708. doi: 10.1111/APHA.13970
[7] Marijn Muurling et al. “Remote monitoring technologies in Alzheimer’s disease: design of the RADAR-AD study”. In: Alzheimer’s Research and Therapy 13.1 (Dec. 2021). issn: 17589193. doi: 10.1186/s13195-021-00825-
[8] Els I.S. Most et al. “Discrepancy between subjective and objective sleep disturbances in early-and moderate-stage alzheimer disease”. In: American Journal of Geriatric Psychiatry 20.6 (June 2012), pp. 460–467. issn: 15457214. doi: 10.1097/JGP.0b013e318252e3ff
[9] Philip Hougaard and Pho@novo Dk. Multi-state Models: A Review. Tech. rep. 1999, pp. 239–264
[10] Luís Filipe Meira-Machado et al. Multi-state models for the analysis of time-to-event data. 2009. doi: 10.1177/0962280208092301
[11] Jennifer G. Le-Rademacher, Terry M. Therneau, and Fang-Shu Ou. “The Utility of Multistate Models: A Flexible Framework for Time-to-Event Data”. In: Current Epidemiology Reports 9.3 (June 2022), pp. 183–189. doi: 10.1007/s40471-022-00291-y
[12] M. W. Johns. “A new method for measuring daytime sleepiness: the Epworth sleepiness scale”. In: Sleep 14.6 (1991), pp. 540–545. issn: 0161-8105. doi: 10.1093/SLEEP/14.6.540
[13] Daniel J. Buysse et al. “The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research”. In: Psychiatry research 28.2 (1989), pp. 193–213. issn: 0165-1781. doi: 10.1016/0165-1781(89)90047-4
[14] Ye Zhang et al. “Sleep in Alzheimer’s disease: a systematic review and meta-analysis of polysomnographic findings”. In: Translational Psychiatry 2022 12:1 12.1 (Apr. 2022), pp. 1–12. issn: 2158-3188. doi: 10.1038/s41398-022-01897-y
[15] Yu Ying Sun et al. “Sleep-Wake Disorders in Alzheimer’s Disease: A Review”. In: ACS chemical neuroscience 13.10 (2022). issn: 1948-7193. doi: 10.1021/ACSCHEMNEURO.2C00097
[16] Xiangyang Xiong et al. “Research advances in the study of sleep disorders, circadian rhythm disturbances and Alzheimer’s disease”. In: Frontiers in Aging Neuroscience 14 (Aug. 2022). issn: 16634365. doi: 10.3389/FNAGI.2022.944283
[17] Elena Urrestarazu and Jorge Iriarte. “Clinical management of sleep disturbances in Alzheimer’s disease: current and emerging strategies”. In: Nature and Science of Sleep 8 (2016), p. 21. issn: 11791608. doi: 10.2147/NSS.S76706